Past Research
Past Research
Our preliminary work was essentially a pilot study in humans performed with IRB approval. We were not looking for clinical efficacy. However, secondary endpoints such as improvement in their conditions were certainly optimistic and have acted as a catalyst for our move to a phase 1/2 trial with the FDA.
Pre-FDA work with stromal vascular fraction (SVF): 33 Patients with various incurable, recalcitrant to all attempted therapies, neurodegenerative diseases had a total of 151 injections with a 3.5-year follow-up. There were minor adverse events (treated and resolved):
Reservoir AEs (per 151 injections):
Clinical Adverse Events (treated and resolved – per 151 injections):
For the neurodegenerative diseases we are targeting, there are currently no real effective therapies. Our revolutionary direct-injection stem cell therapy offers patients suffering from Alzheimer’s disease, ALS, and Multiple sclerosis, a new approach. The following are some of our clinical efficacy results.
Fig. 1 below shows the bad Tau protein, P-Tau, diminishing, in injected Alzheimer’s patients
Fig. 2 below shows the injection technique
Fig. 3 below shows one of our Alzheimer’s patients exhibiting actual growth of the hippocampus, the “short-term memory area of the brain”

Fig. 1

Fig. 2
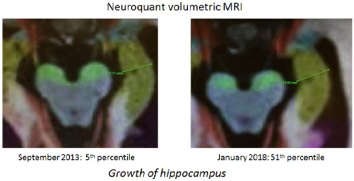
Fig. 3
Alzheimer’s Disease: 12 Treated
- 5 not declining and getting better
- 6 not declining
- 1 worse, since stopping treatment
Multiple Sclerosis: 6 Treated
- 3/6 subjects improving
- 3/6 not declining
Traumatic Brain Injury: 1 Treated
- 1/1 subject improving

A Typical Result for One of Our Alzheimer's Patients


